Syringe Testing to ISO 11040
MECHANICAL TESTING OF PREFILLED GLASS SYRINGES
ISO 11040 is a testing standard that addresses the design and functional properties of prefilled syringes. ISO 11040 is used primarily within the pharmaceutical industry and is critical for ensuring that syringes work properly in a clinical setting. ISO 11040 evaluations are performed throughout the manufacturing process to minimize any chance of defective equipment leaving the factory as failure of any subcomponent can have a major impact on either the physician or patient: an improper seal can cause oxidation of the drug and affect the shelf life of the product, while structural damage to the syringe barrel could lead to device failures. Note: Labs that manufacture syringes must be in compliance with 21 CFR Part 11.

NAVIGATING THE STANDARD
ISO 11040 is composed of 8 parts:
Parts 1-3 refer to the subcomponents of cartridges used for local anesthetic in dental settings.
Parts 4-6 refer to the subcomponents of prefilled syringe including glass barrels, plastic barrels, and plunger stoppers.
Part 7 addresses the packaging systems typically used for pre-sterilized fill and finish applications. "Fill and finish" refers to the process of preparing the syringes for their end use, which is often a bottleneck in the supply chain.
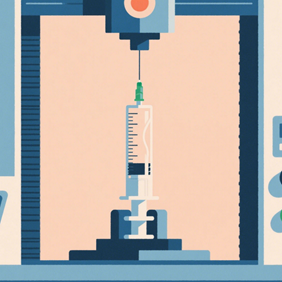
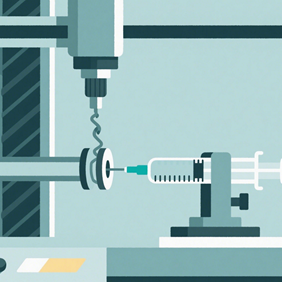
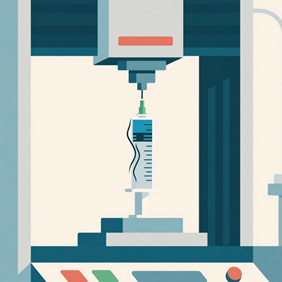
Part 8 outlines the test methods and evaluation criteria for the finished syringe rather than the individual subcomponents. These tests include durability of the syringe barrel, functional use of the devices (break loose and glide forces, needle penetration forces), and evaluation of the device closures and safety features. Many of the test methods outlines in part 4 and part 6 are analogous to those in part 8.


























